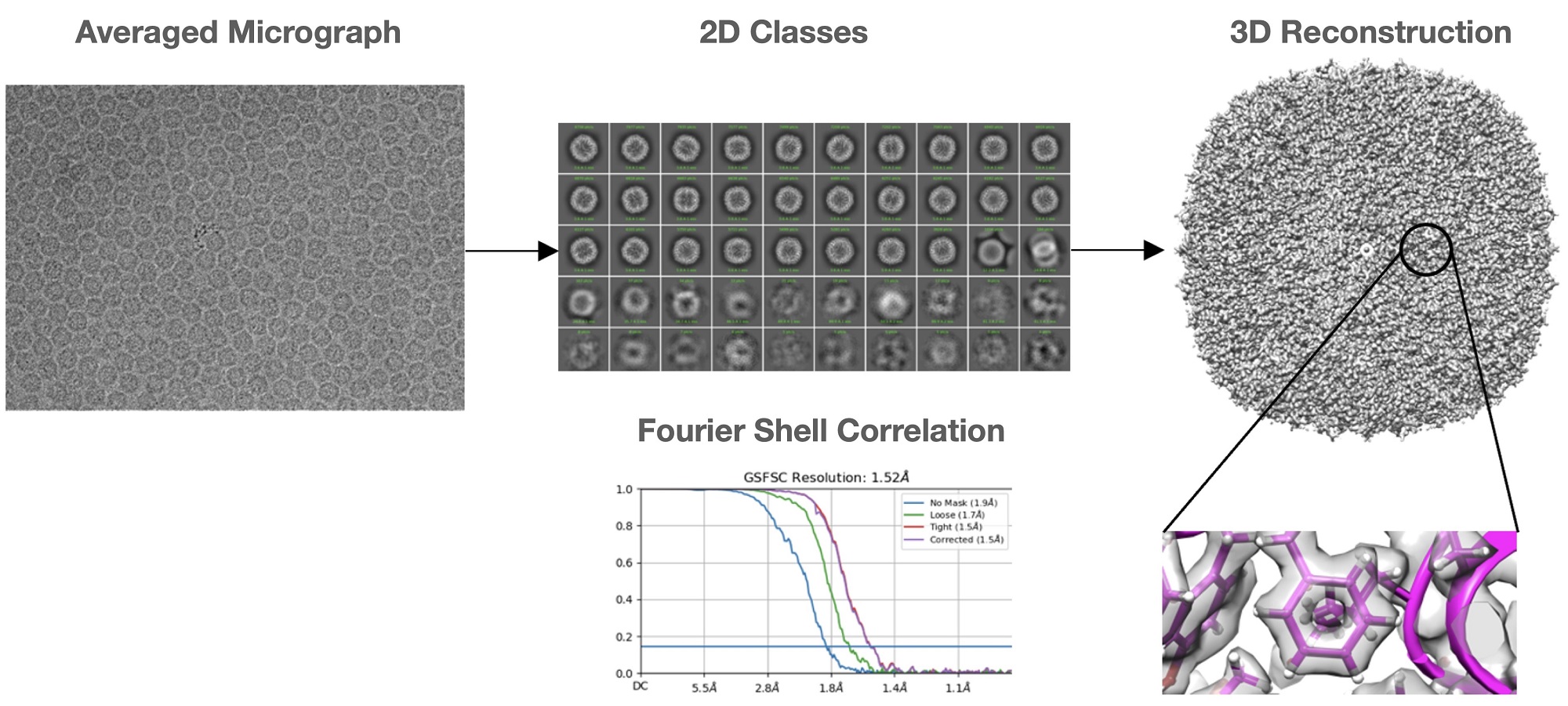
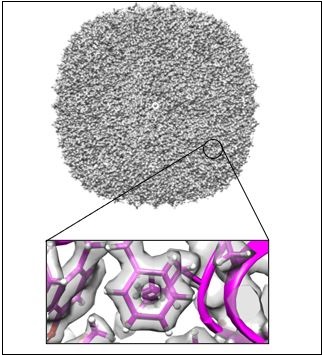
 Earlier this year CMM teamed up with researchers from UQ’s School of Chemistry and Molecular Biosciences and application engineers from JEOL to achieve an important milestone. During a short test of UQ’s JEOL CRYO ARM™300 transmission electron microscope, almost 2000 micrographs containing pictures of the small iron storage protein, ferritin, were recorded over a period of 5 hours. Images such as these are routinely used around the world to reveal incredibly detailed 3-dimensional structures of proteins through a process known as single particle analysis. In this case, the images recorded by the team led to a 3D structure of ferritin being determined at a resolution of 1.5 Å, making it the highest resolution structure solved at UQ by this technique, and one of the highest resolution structures reported worldwide.
Earlier this year CMM teamed up with researchers from UQ’s School of Chemistry and Molecular Biosciences and application engineers from JEOL to achieve an important milestone. During a short test of UQ’s JEOL CRYO ARM™300 transmission electron microscope, almost 2000 micrographs containing pictures of the small iron storage protein, ferritin, were recorded over a period of 5 hours. Images such as these are routinely used around the world to reveal incredibly detailed 3-dimensional structures of proteins through a process known as single particle analysis. In this case, the images recorded by the team led to a 3D structure of ferritin being determined at a resolution of 1.5 Å, making it the highest resolution structure solved at UQ by this technique, and one of the highest resolution structures reported worldwide.
In a true team effort, Dr Naphak Modhiran and Dr Yu Shang Low from UQ SCMB purified and supplied the protein, which was then frozen and imaged by Dr Makino Fumiaki (JEOL) and Dr Lou Brillault (CMM). Processing of the data to produce the highly detailed 3-dimensional structures was then performed by Mr Matthew Jackman at UQ SCMB with the team of researchers at UQ SCMB also involving Associate Professors Michael Landsberg and Daniel Watterson, who supervised the work.
The achievement sets a new benchmark for the cryo-EM community of The University of Queensland and highlights that JEOL’s cryo-EM technology platforms, which UQ was an early adopter of, can deliver outputs that match world benchmarks. The 3D structure that was solved as part of this test has a resolution which is exceeded by only one other structure solved using this technique in combination with a JEOL CRYO ARM™ instrument, and positions the UQ facility as one of only a handful of institutions worldwide who have reported single particle cryo-EM structures at this resolution. Structures that reach these benchmarks are of increasingly important biological significance. They allow for complete atomic models to be interpreted and enable applications such as observing the way that small drugs and drug-like molecules bind to biomedically important proteins, or understanding the chemical basis of enzymatic activities.